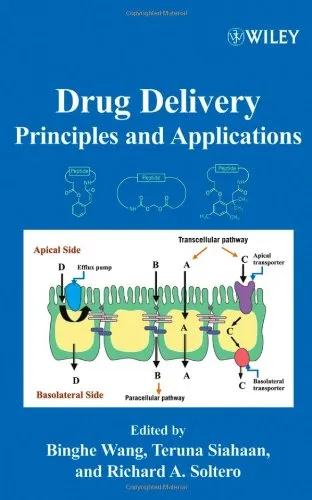
Drug Safety Evaluation: Methods and Protocols
4.0
Reviews from our users

You Can Ask your questions from this book's AI after Login
Each download or ask from book AI costs 2 points. To earn more free points, please visit the Points Guide Page and complete some valuable actions.Related Refrences:
Introduction to "Drug Safety Evaluation: Methods and Protocols"
"Drug Safety Evaluation: Methods and Protocols" is a comprehensive guide aimed at providing a meticulous understanding of the experimental techniques and protocols used in assessing the safety of pharmaceutical products. This book presents an extensive exploration of methods and research tools that are pivotal to ensure drugs are safe for human use, thereby aiding scientists, researchers, and healthcare professionals in their critical responsibilities.
The pharmaceutical industry is fundamentally driven by innovation balanced with rigorous testing to guarantee safety and efficacy. This book stands as an essential resource, laying out a systematic approach to drug safety evaluation and emphasizing its multidisciplinary nature. Edited by Jean-Charles Gautier and authored by Alberto Lodola, alongside contributions from experts in the field, this book merges theory with practical application, offering both depth and clarity. Let’s delve deeper into what this book provides, why it is significant, and what readers can expect to take away from it.
Detailed Summary
Safety evaluation is a cornerstone of the drug development pipeline, involving clinical and non-clinical methodologies to anticipate potential risks before administering medication to humans. This book brings together a wide range of protocols, from in vitro and in vivo assays to computational simulation tools, which collectively contribute to this evaluation.
The content is structured logically, with an initial focus on preclinical safety testing and regulatory requirements. This is followed by protocols aligned with ethical considerations, safety pharmacology, and toxicological testing. Advanced sections of the book introduce high-throughput screening techniques and predictive toxicology, ensuring experts have access to the most recent advancements in the field.
Particular attention is given to the integration of computational tools, including molecular dynamics and predictive modeling, which are transforming how drug safety is analyzed in modern research. Each protocol is presented with clarity, featuring details on reagents, equipment, step-by-step workflows, and troubleshooting tips.
The goal is not merely to offer procedures but to promote the understanding of why these protocols are vital. This combination of knowledge transfer and practical guidance ensures this book is an indispensable asset for laboratory researchers, toxicologists, regulatory professionals, and pharmaceutical scientists.
Key Takeaways
- Comprehensive coverage of traditional and modern drug safety evaluation methods.
- Detailed guidance on in vitro, in vivo, and in silico toxicity testing protocols.
- Practical insights into high-throughput screening and advanced computational modeling.
- Step-by-step experimental workflows with troubleshooting sections.
- Focused discussions on ethical and regulatory considerations for drug safety assessment.
Readers will gain both technical skills and a strong foundation in the key principles and challenges of drug safety evaluation, which is critical for pharmaceutical innovation and public health protection.
Famous Quotes from the Book
"The complexities of drug discovery and development demand safety protocols that are not only scientifically rigorous but also ethically sound."
"In the chase for innovation, we must never lose sight of the responsibility to minimize harm and ensure human safety."
Why This Book Matters
The field of pharmaceutical development is rapidly evolving, with new technologies, data frameworks, and regulatory landscapes emerging each year. Despite these advancements, the safety of medication will always remain paramount. This book plays a pivotal role in equipping professionals with the knowledge and tools necessary to navigate the increasingly complex demands of drug safety evaluation.
For healthcare professionals, "Drug Safety Evaluation: Methods and Protocols" fosters a deeper appreciation for the risks involved in drug development and the mechanisms put in place to mitigate them. For researchers and industry experts, it provides actionable insights and step-by-step guidance that are indispensable to experimental and computational drug safety testing.
Perhaps most importantly, this book underscores the ethical imperative of drug safety evaluation: protecting human lives while advancing medical science. Whether you are a student entering the field, an experienced researcher, or a policy maker involved in pharmaceutical regulation, this resource is invaluable. Structured to blend theory with practice, it ensures a lasting impact on both individual careers and the collective progress of drug safety research.
Free Direct Download
You Can Download this book after Login
Accessing books through legal platforms and public libraries not only supports the rights of authors and publishers but also contributes to the sustainability of reading culture. Before downloading, please take a moment to consider these options.
Find this book on other platforms:
WorldCat helps you find books in libraries worldwide.
See ratings, reviews, and discussions on Goodreads.
Find and buy rare or used books on AbeBooks.
1354
بازدید4.0
امتیاز0
نظر98%
رضایتReviews:
4.0
Based on 0 users review
Questions & Answers
Ask questions about this book or help others by answering
No questions yet. Be the first to ask!