Drug Product Development for the Back of the Eye (AAPS Advances in the Pharmaceutical Sciences Series, 2)
4.0
Reviews from our users

You Can Ask your questions from this book's AI after Login
Each download or ask from book AI costs 2 points. To earn more free points, please visit the Points Guide Page and complete some valuable actions.Related Refrences:
Introduction to "Drug Product Development for the Back of the Eye"
"Drug Product Development for the Back of the Eye," part of the esteemed AAPS Advances in the Pharmaceutical Sciences Series, offers a comprehensive guide into the development of therapies targeting posterior segment eye diseases. As the burden of diseases such as age-related macular degeneration, diabetic retinopathy, and retinal degeneration continues to grow worldwide, this book serves as a crucial resource for pharmaceutical scientists, researchers, clinicians, and innovators working to design effective treatments.
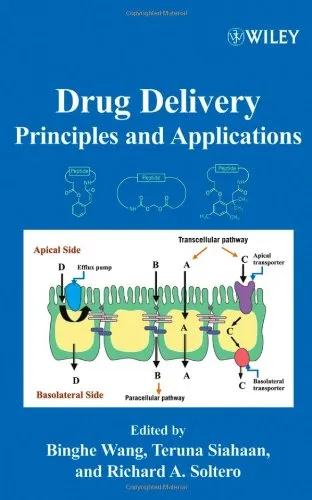
The anatomy and physiology of the eye's posterior segment present unique challenges for drug delivery. Factors such as tight ocular barriers, limited vascularization, and the need for localized, prolonged treatment demand innovative approaches to drug development. This book delves deeply into these complexities, offering not only detailed insights into disease mechanisms but also state-of-the-art techniques for formulating, testing, and delivering drugs effectively to the back of the eye. By covering a breadth of topics, including preclinical studies, regulatory considerations, and clinical translations, the text bridges theoretical knowledge with practical application.
Detailed Summary of the Book
The field of ophthalmology has witnessed significant advancements over the past few decades, thanks to innovations in drug delivery technologies and diagnostic techniques. However, treating the posterior segment of the eye remains a challenging frontier. This book addresses those challenges comprehensively by organizing its contents into sections that gradually build an understanding of drug development tailored for the back of the eye.
In its early chapters, the book introduces readers to the anatomy and physiology of the eye, emphasizing aspects unique to the posterior segment. This lays the groundwork for understanding the barriers to effective drug delivery. Subsequent chapters focus on disease-specific considerations, presenting the pathophysiology and progression of common conditions such as macular degeneration, diabetic retinopathy, and uveitis. It then transitions into discussing strategies for drug development, covering innovative drug delivery systems (e.g., intravitreal injections, implants, and nanoparticles), the pharmacokinetics of ocular drugs, and novel therapeutic approaches like gene therapy.
Importantly, the book incorporates insights into the regulatory landscape and clinical trial design, offering essential guidance for navigating the process of drug development, from preclinical studies to product registration. Practical case studies and examples from industry and academia enrich the content, giving readers a well-rounded perspective on what successful product development requires.
Key Takeaways
- Understanding the intricate anatomy and physiology of the posterior segment of the eye and its implications for drug delivery.
- An in-depth analysis of the diverse drug delivery systems used for targeting back-of-the-eye diseases, including emerging technologies like nano-based carriers.
- Insights into pathophysiology and therapeutic strategies for common posterior eye conditions, including diabetic retinopathy, macular degeneration, and beyond.
- A guide to regulatory hurdles and considerations in ocular drug development, equipping readers with practical knowledge essential for advancing treatments to market.
- Practical advice and case studies detailing successful strategies in product design, preclinical testing, and clinical application.
Famous Quotes from the Book
"Understanding the eye as a system is a key prerequisite to designing effective therapies. Bridging science with application paves the way for meaningful treatment options that improve patients' lives."
"Innovation in back-of-the-eye drug delivery is not merely about overcoming barriers; it is about redefining what is possible for patients with previously untreatable ocular diseases."
Why This Book Matters
Posterior segment eye diseases are among the leading causes of vision impairment and blindness globally, presenting a growing socioeconomic and clinical challenge. Despite their significance, developing effective therapies for these conditions is notoriously difficult due to the complex structure and limited accessibility of the eye’s posterior segment. "Drug Product Development for the Back of the Eye" fills this critical void by equipping readers with the knowledge, tools, and insights needed to address these challenges.
This book matters because it not only outlines the current state of the art for back-of-the-eye drug development but also serves as an inspiration for researchers and innovators to push the boundaries of what can be achieved. By blending comprehensive scientific content with pragmatic guidance, it appeals to a wide spectrum of professionals, from laboratory scientists to regulatory personnel and clinicians.
The collaborative perspectives from leading experts around the world ensure that this book provides a truly global view of ocular drug development. Whether you're an aspiring researcher, a seasoned pharmaceutical scientist, or a policymaker in ophthalmology, the content of this text offers invaluable insights for improving patient outcomes while advancing the field of ocular therapeutics.
Free Direct Download
You Can Download this book after Login
Accessing books through legal platforms and public libraries not only supports the rights of authors and publishers but also contributes to the sustainability of reading culture. Before downloading, please take a moment to consider these options.
Find this book on other platforms:
WorldCat helps you find books in libraries worldwide.
See ratings, reviews, and discussions on Goodreads.
Find and buy rare or used books on AbeBooks.
1300
بازدید4.0
امتیاز0
نظر98%
رضایتReviews:
4.0
Based on 0 users review
Questions & Answers
Ask questions about this book or help others by answering
No questions yet. Be the first to ask!